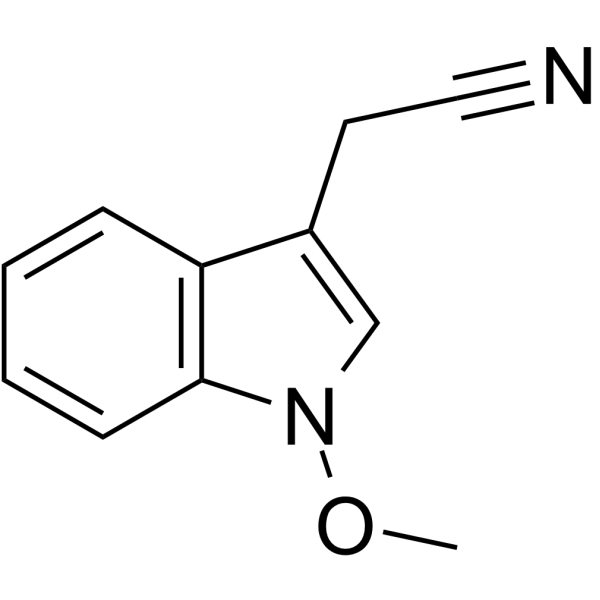
Caulilexin C
CAS No. 30536-48-2
Caulilexin C( —— )
Catalog No. M29109 CAS No. 30536-48-2
Caulilexin C shows inhibitory activity on human Acyl CoA: cholesterol transferase I (hACATI) and on human Acyl CoA: cholesterol transferase 2 (hACAT2) at 100 mug/ml.
Purity : >98% (HPLC)
 COA
COA
 Datasheet
Datasheet
 HNMR
HNMR
 HPLC
HPLC
 MSDS
MSDS
 Handing Instructions
Handing Instructions
| Size | Price / USD | Stock | Quantity |
| 5MG | 335 | Get Quote |


|
| 10MG | 492 | Get Quote |


|
| 25MG | 781 | Get Quote |


|
| 50MG | 1053 | Get Quote |


|
| 100MG | Get Quote | Get Quote |


|
| 200MG | Get Quote | Get Quote |


|
| 500MG | Get Quote | Get Quote |


|
| 1G | Get Quote | Get Quote |


|
Biological Information
-
Product NameCaulilexin C
-
NoteResearch use only, not for human use.
-
Brief DescriptionCaulilexin C shows inhibitory activity on human Acyl CoA: cholesterol transferase I (hACATI) and on human Acyl CoA: cholesterol transferase 2 (hACAT2) at 100 mug/ml.
-
DescriptionCaulilexin C shows inhibitory activity on human Acyl CoA: cholesterol transferase I (hACATI) and on human Acyl CoA: cholesterol transferase 2 (hACAT2) at 100 mug/ml.(In Vitro):Toward this end, the metabolism of indolyl glucosinolates, their corresponding desulfo-derivatives, and derived metabolites, by three fungal species pathogenic on crucifers was investigated. While glucobrassicin, 1-methoxyglucobrassicin, 4-methoxyglucobrassicin were not metabolized by the pathogenic fungi Alternaria brassicicola, Rhizoctonia solani and Sclerotinia sclerotiorum, the corresponding desulfo-derivatives were metabolized to indolyl-3-acetonitrile, Caulilexin C (1-methoxyindolyl-3-acetonitrile) and arvelexin (4-methoxyindolyl-3-acetonitrile) by R. solani and S. sclerotiorum, but not by A. brassicicola. That is, desulfo-glucosinolates were metabolized by two non-host-selective pathogens, but not by a host-selective. Indolyl-3-acetonitrile, Caulilexin C and arvelexin were metabolized to the corresponding indole-3-carboxylic acids. Indolyl-3-acetonitriles displayed higher inhibitory activity than indole desulfo-glucosinolates. Indolyl-3-methanol displayed antifungal activity and was metabolized by A. brassicicola and R. solani to the less antifungal compounds indole-3-carboxaldehyde and indole-3-carboxylic acid.
-
In VitroCaulilexin C causes complete growth inhibition (0.5 mM) of Rhizoctonia solani and has a smaller effect on Leptosphaeria maculans (77% inhibition), appearing to be slightly more antifungal than arvelexin.
-
In Vivo——
-
Synonyms——
-
PathwayOthers
-
TargetOther Targets
-
Recptor——
-
Research Area——
-
Indication——
Chemical Information
-
CAS Number30536-48-2
-
Formula Weight186.214
-
Molecular FormulaC11H10N2O
-
Purity>98% (HPLC)
-
SolubilityIn Vitro:?DMSO : 100 mg/mL (537.03 mM)
-
SMILESCOn1cc(CC#N)c2ccccc12
-
Chemical Name——
Shipping & Storage Information
-
Storage(-20℃)
-
ShippingWith Ice Pack
-
Stability≥ 2 years
Reference
molnova catalog



related products
-
Astegolimab
Astegolimab (RG 6149) is a humanised IgG2 monoclonal antibody that targets the IL-33 receptor and inhibits IL-33 signalling. Astegolimab is used in the study of chronic obstructive pulmonary disease (COPD) and severe asthma in adults.
-
Humantenirine
Humantenirine is an indole alkaloid isolated from Gelsemium sempervirens.
-
Lys-[Des-Arg9]Bradyk...
Lys-[Des-Arg9]Bradykinin,TFA is Endogenous potent and highly selective bradykinin B1 receptor agonist (Ki values are 0.12 and > 30000 nM at human B1 and B2 receptors respectively).



 Cart
Cart
 sales@molnova.com
sales@molnova.com


